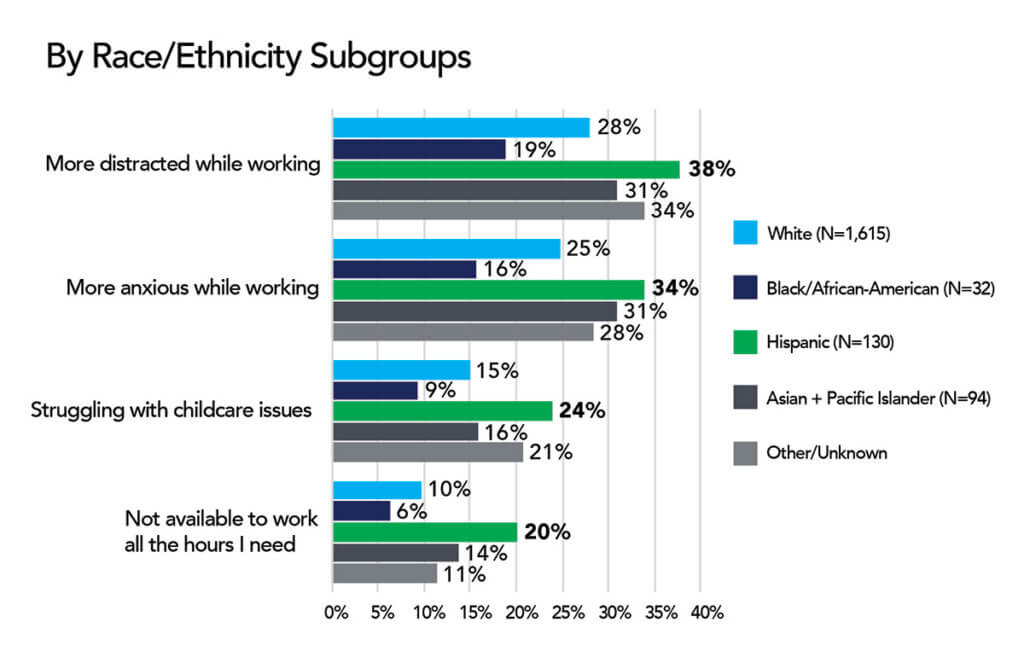
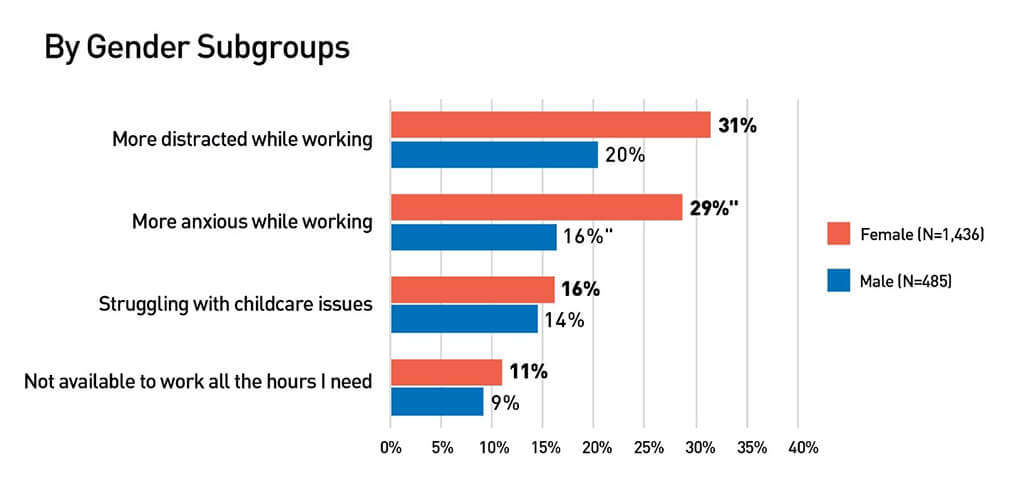
For the 40% of all U.S. households with children under 18, the pandemic is posing significant challenges as schools and daycares close, change schedules, or switch to virtual formats. But for healthcare workers, these changes in school schedules and formats can cause extra challenges and make the balancing act between work and home even harder.
We asked HERO members how school changes were affecting their lives. More than 2,000 members replied to our questions reporting that 28% feel more distracted at work, 25% are feeling more anxious because of school changes. In addition, 15% are struggling to find and pay for childcare, and 10% are not able to work the number of hours they need to work.
But even those who do not have children at home report that they are affected through extra shifts, feeling at risk caring for grandchildren, or managing their teams.
One HERO member said: “I don’t have children, but as a nursing executive I see the impact it has on the staff and our ability to staff our units. The core staff are more stressed, and our per diem staff are hesitant to pick up time because they don’t know if the schools will be making last minute changes and sending the kids home. It’s very challenging for the staff and the managers who want to support their staff but also need to meet the operational needs of their departments.”
Another added, “I am not a parent or caregiver; however, I am responsible for taking on extra responsibilities that my coworkers with children are less able to. While I’m trying to help, it’s adding to my burnout.”
There’s another hidden toll as many clinician-parents are trying to help their friends and fellow parents navigate the pandemic as well.
“As both a parent and a medical professional, I am completely exhausted by the dozens of ‘just one quick question’ calls I am getting from fellow parents who desperately want and need advice and reliable information, but don’t have a functional relationship with a healthcare provider of their own. Trying to find a healthy balance between keeping my work professional, maintaining boundaries for my own well-being, and supporting my community is a HUGE source of exhaustion, anxiety, distress, burnout and constant self-doubt in my life,” reported one HERO member.
For those who are able to have a full-time parent present to take care of school and daycare closures, loss of income or opportunity is another challenge: “Because my husband lost his job due to COVID-19, childcare isn’t an issue, but I am seriously stressed about the loss of income,” one HERO member said.
Many reported having less time for themselves as they rearrange work schedules to be home with children:
- “I am working nights and weekends to help my children with e-learning and I am exhausted.”
- “I have to now work nearly every weekend in order to be home during the week for virtual schooling.”
- “I have to rearrange my work schedule to be home more during the week. I am working every weekend for the next ten weeks, at a minimum.”
For those who are not able to change their schedule, finding a work-life balance is hard as well: “I feel guilty that I’m not able to provide emotional and educational support for my child because I am working full time and longer days than ever. I’m a manager as well, so I am very stressed about my staff’s ability to work due to their children’s educational issues,” said one HERO member.
Many families of healthcare workers are struggling financially to manage school changes: “We have significant increases in childcare and education costs as we both are unable to work from home. To avoid loss of income and leave from work we have transferred our school age children into private school so they can attend in-person and are paying significantly more for childcare arrangements after school,” said one HERO member.
For those healthcare workers living in places where schools did not change schedules, the fear of change is significant and the normal schedule can cause disruption as well: “My wife has stopped watching our school-aged grandchild due to fear of them contracting COVID-19 from her since she is with other children in the classroom,” said one HERO member.
Many respondents said that while they do not have school aged children, they face other challenges such as caring for special-needs adults and children managing remote college.
These stressors are not unique to HERO members of course, but they do put extra stress on an already overly burdened healthcare system.
“A major goal of the HERO Registry is to listen to our members and give a larger voice to their experiences during the pandemic. School and child care changes are affecting all workers with young children at home,” said Emily O’Brien, principal investigator for the HERO Registry. “But for healthcare workers, these struggles may have a larger impact on the overall healthcare system. It’s important for leaders to hear these perspectives and understand their impact.”
As the nation heads into flu season on top of the COVID-19 pandemic, a strong healthcare workforce will depend on finding answers to safely provide childcare.
We appreciate the input from HERO Registry members who completed this survey. Your feedback helps bring awareness to the challenges healthcare workers are facing and guide future research needs. Our next topic will be on the financial impact of the pandemic.
If you have not joined HERO, sign-up today. Together through research we can identify solutions to help protect healthcare workers.